
Tytus Technology Service LLC was founded in 2019 with a mission to provide specialized services and regulatory consulting to globe clients in dental, pharmaceutical, nutraceutical, dietary supplement and food industry.

Tytus Technology Service LLC was founded in 2019 with a mission to provide specialized services and regulatory consulting to globe clients in dental, pharmaceutical, nutraceutical, dietary supplement and food industry.



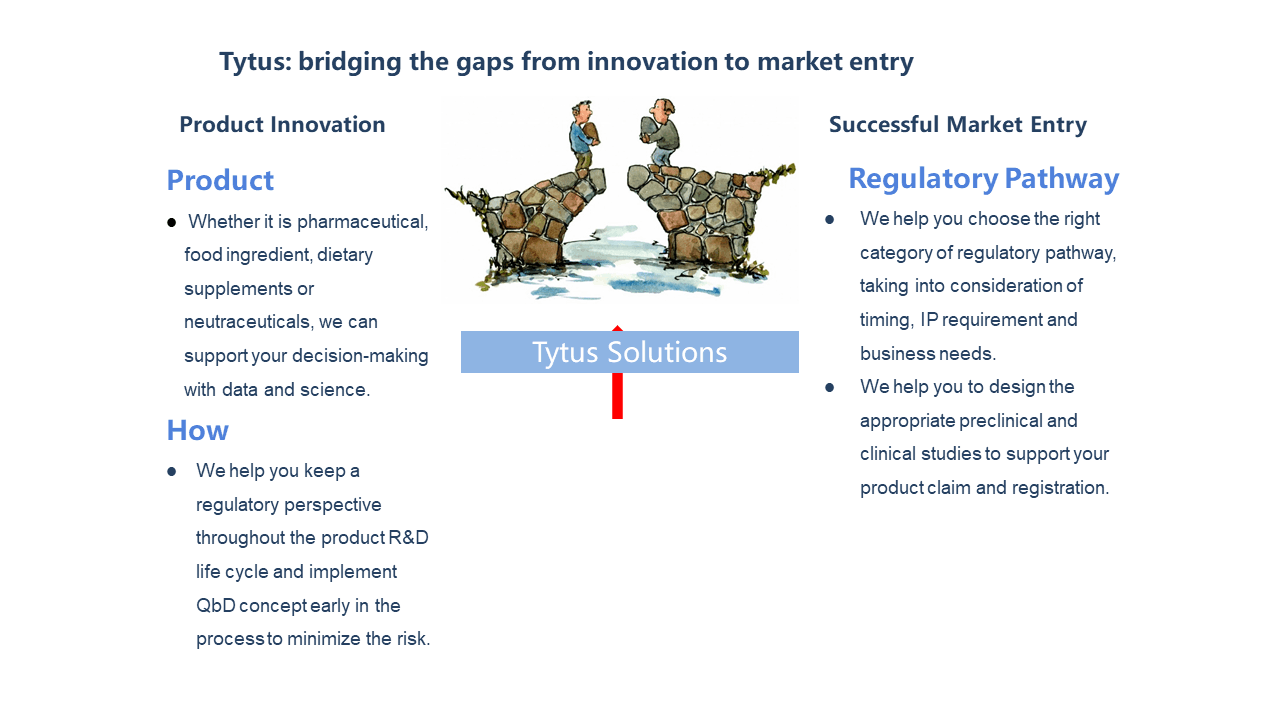
Helping our clients selecting the best regulatory filing route (such as IND, NDA, ANDA, GRAS, NDIN) , then working with the clients to prepare dossiers and provide step-by-step guidance that leads to successful filing.
Supporting our client’s regulatory filing with appropriately designed study protocols, assisting and supervising preclinical and toxicology studies and guidance for study reporting.
Supporting our clients with the most appropriate regulatory filing, and compiling dossiers to file for FDA 510K, medical device, cosmetics, claims and labels compliance consulting.
We have experience and networks with American Dental Association (ADA) to help your dental products getting the prestigious ADA seal of approval, to support your marketing globally with this prestigious certification.
We have expert resources to form independent GRAS panels to verify through scientific procedures that your product meets the “Generally Regarded As Safe" definition.
We partner with many GLP labs in Europe, China, USA providing human cells, and animal cells for the prediction of toxic and long-term effects of agents on the human body and the environment. Our cell culture systems are important for your day-to-day work. Human and non-human hepatocytes are available as fresh or cryopreserved.
Product Category:
Cell and Tissue Products
Liver products
Human liver cells
Animal liver cells
Liver subcellular fractions
Skin products
Skin tissues
Skin discs & dermatomed skin
Fibroblasts
Keratinocytes
Other organs and body fluids
Trout S9 fraction: intestine, gill, brain
Consumables
Cell Culture Media
3D Hepatocyte Maintenance Medium
Long Term Hepatocyte Culture Medium
Short Term Hepatocyte Culture Medium
Thawing and Plating Kits
Collagen products
ULA plates
AI Website Generator